An electrochemical cell converts chemical energy into electrical energy (or vice versa). In the case where electrical energy is generated (Galvanic cell), it is driven by a pair of spontaneous reduction-oxidation (redox) reactions. These reactions take place at the two electrodes, which are in contact with an electrolyte, forming two half-cells. An electrolyte is essentially a solution which is overall electrically neutral, but is electrically conducting because it contains ions, which are atoms or molecules with electrical charges on them. The half-cells are connected with a salt bridge, or else are separated by a semi-permeable membrane.
At one of the electrodes, positively charged ions (cations) will gain electrons in a reduction reaction. This electrode is therefore known as the cathode. The electrode where negatively charged anions will give up their extra electrons in an oxidation reaction is known as the anode. Electrons picked up by the anode pass through an external circuit to perform work and then arrive at the cathode, completing the circuit.
Now it’s easy enough to remember that cations go to the cathode and anions go to the anode, but what about the other stuff? There are some helpful mnemonics that can be used to keep track of the rest. C and R are consonants, and so we can remember that the the cathode is where the cations get reduced. A and O, on the other hand, are vowels, and thus the anode is where anions get oxidized. Furthermore, we can think of “reduction” as becoming more negative, and thus remember that reduction is the process by which negatively charged electrons are gained. From that we can gather that cations are the positively charged ions. Likewise, oxidation, the opposite process of reduction, is where electrons are lost, and thus recall that anions are the negatively charged ions.
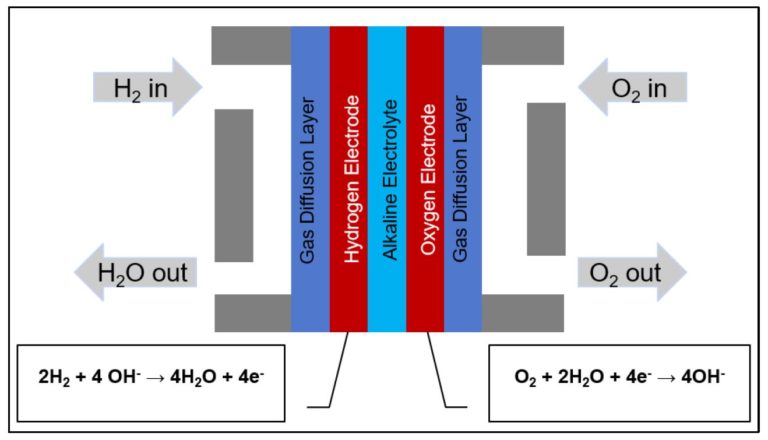
Figure 1: Cartoon schematic of alkaline fuel cell
In a hydrogen fuel cell, hydrogen is fed into the cell, where it reacts with atmospheric oxygen, producing water and electricity. In an alkaline fuel cell, an alkaline solution such as potassium hydroxide (KOH) is used as the electrolyte. At the anode, the hydrogen reacts with hydroxide ions in the following oxidation reaction:
H2 + 2OH– → 2H2O + 2e–
This is the half-reaction in which the water is produced, while the electrons from this reaction pass through the external circuit to the cathode. Once there, the electrons participate in the reduction of the oxygen, as follows:
½ O2 + H2O + 2e– → 2OH–
Combining these two half-reaction, it can be seen that we get the following overall reaction:
H2 + ½ O2 → H2O
A cartoon schematic of an alkaline fuel cell and its operation are provided above.
