One of the critical challenges facing fuel cells is degradation of the catalyst/support structure. A major mechanism of degradation is air/air (cathode/anode) startup/shutdown (SU/SD) degradation. Over time it can lead to significant corrosion of the carbon support structure, which in turn can lead to a loss of porosity of the catalyst layer, as well as detachment of the catalyst particles. Thus SU/SD degradation can greatly reduce fuel cell lifetime.
SU/SD degradation occurs at the startup of the fuel cell after it has been stopped for a prolonged period. While the fuel cell has been shut down, air has leaked over to the anode from the cathode or the surrounding environment. This results in the formation of two different regions within the anode: a hydrogen region near the H2 inlet and an oxygen region near the outlet.
At startup, a voltage difference is established between the cathode and the anode, with different reactions occurring at the two electrodes. At the air cathode the reaction taking place is the oxygen reduction reaction (ORR). At the anode, under normal conditions in which it is supplied with hydrogen and oxygen is absent, the hydrogen oxidation reaction (HOR) takes place. Within the portion of the anode that isn’t starved for hydrogen, this is indeed what takes place.
Due to their being electrically connected, the portion of the anode that isn’t starved for hydrogen (and can thus undergo HOR) imposes the same potential difference between the hydrogen starved region of the anode and the cathode. Because of the absence of hydrogen, however, HOR cannot take place there, and thus with the availability of oxygen, ORR takes place at the anode instead. This reaction takes place at a higher voltage than HOR. Thus, in order to maintain the same potential difference between the electrodes, the voltage in is driven up in the portion of the cathode corresponding to the hydrogen starved region of the anode.
At these higher potentials, the cathode undergoes water oxidation and carbon corrosion, forming hydrogen and CO2. Thus, the carbon support structure in the cathode that is typically used in fuel cells is degraded during the initial startup, before the hydrogen has fully permeated the anode. Figure 1 provides a representation of the change in potentials and reactions within the cathode and anode.
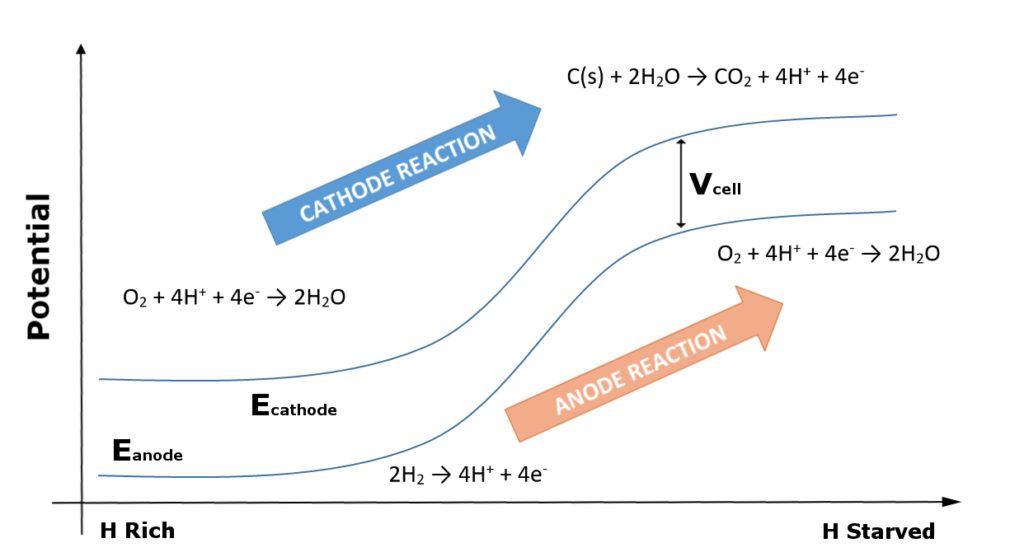
Figure 1: Change in electrode potentials and reactions going from relatively hydrogen rich to hydrogen starved regions of the anode.
There are a few different general approaches that can be taken in order to try to tackle the problem of SU/SD degradation. Perhaps the most obvious is the development of an alternative to the traditional carbon support structure that will not corrode at the higher potentials caused by ORR taking place at the anode. Another approach would be to incorporate a catalyst into the cathode that promotes the harmless water oxidation process over carbon corrosion. Lastly, the development of an HOR selective catalyst that would suppress ORR at the anode, thus preventing the potential from being driven up at the cathode.
